Research


Home > Research > MAEYAMA laboratory
Research fields/Keywords:polymer synthesis (polycondensation); polymerization reactions, ion collectors, and highly active Pd catalysts; solvent-soluble aromatic polyketones, and cured polyketones; heat-resistant, transparent heat-resistant, and luminescent heat-resistant materials
 Professor Katsuya MAEYAMA
Professor Katsuya MAEYAMA


Aromatic polyketones are composed of aromatic rings, ketone carbonyl groups, and ether units and have been attractive as high performance organic materials with high heat-resistance, high chemical resistance, and excellent mechanical properties. As almost all of the commercially available aromatic polyketones such as PEEK and PEKK are insoluble in typical organic solvents, polymer forming via wet process such as spin coating and solvent casting is impossible, which restricts the various application of the aromatic polyketones.
We are developing heat-resistant and organosoluble aromatic polyketones through suitable molecular designing, i.e., introduction of aromatic ring-assemblies with suitable twisting structures such as 2,2'-dialkoxy-5,5-biphenylene and 1,1'-binaphthyl-6,6'-ene units. In addition, we are developing heat-resistant and water-soluble polyketones by introducing hydrophilic units in the side chains, which are potentially applicable to water paints.
The “Suzuki-Miyaura coupling reaction”, developed by Prof. Akira Suzuki, is widely used for synthesizing functional materials because of the ease of handling of reactants and high reactivity. However, the reactivity is not sufficient for the synthesis of aromatic polyketones and hence, obtaining high molecular weight polymer is not possible. Collaborating with the Sakurai group, Osaka University, our group developed a high speed, high activity polymerization catalyst that can be utilized in the Suzuki-Miyaura coupling polymerization. Using the catalyst we developed, the polymerization can be completed within approximately 1 hour. Our current research is targeting further increase in the reactivity. In addition, we are attempting to extend this synthesis technique to other systems such as Mizoroki-Heck polymerization and Sonogashira coupling polymerization.
While the aromatic polyketones developed by our group (1.) have both excellent heat resistance and sufficient solubility, their color is either yellow or pale brown. To meet the exceedingly high societal demand for transparent heat-resistant film, our research is aimed at the development of transparent materials on the basis of aromatic polyketones. By introducing various molecular units, we have developed materials with significantly enhanced transparency when compared to the currently existing aromatic polyketones. Currently, our investigations are targeting further enhancement of the transparency and improvement of various physical properties originating from the optical nature of the materials. In addition, by elucidating why polyketones are ocher colored through fundamental research, we are attempting a novel approach to the development of transparent materials.
We are currently working on utilizing the soluble aromatic polyketones as coatings. To this end, we are developing unique cured systems that can insolubilize polyketones by post processing such as heat, light, and chemical treatment after coating on substrates.
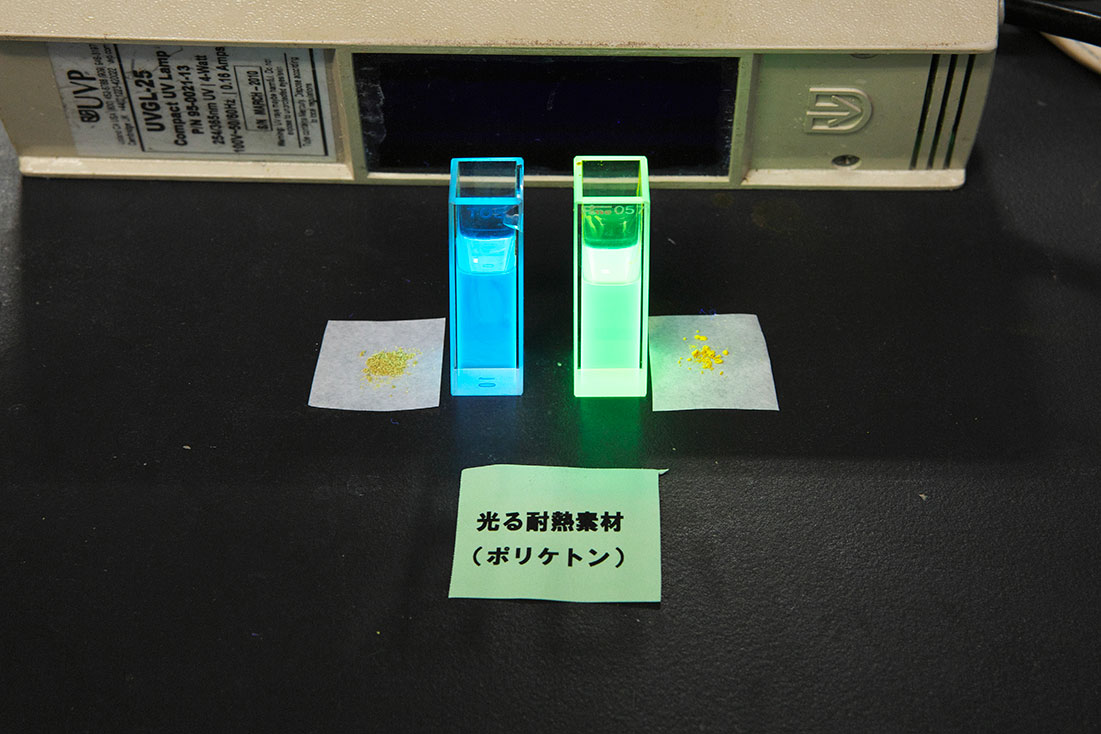
By introducing π-conjugated structures to the aromatic polyketone backbones using the highly active polymerization catalyst developed by our group (2.), we have developed blue~orange emitting materials. Through investigations of the optical and fluorescence characteristics of cross-conjugated polymers obtained by introducing ketone carbonyl or ether oxygen groups suitably into the main chains, we are currently attempting to improve the optical and fluorescent properties of these materials.
While a great many metal ion collectors have been developed, only a few such examples for anions are known. In pursuit of collection behavior, we are producing organic polymer gels endowed with collection sites. We have developed these organic polymer gels through radical copolymerization and polyaddition of monomers possessing collection sites, base monomers, and crosslinking agents. Recently, we have been developing collectors for fluoride ions. Even among collectors, these have recently been growing in importance industrially and environmentally. Using our polymer gels, it is possible to efficiently separate out only the F ions even from a solution where F, Cl and Br ions coexist. Currently, we are attempting to improve the molecular designing such that the polymer gel is fit for practical applications.